Recently, Professor Minghua Huang’s research team from the School of Materials Science and Engineering at Ocean University of China has made significant progress in the development of materials for electrochemical (seawater) hydrogen production. By employing a defect-driven stepwise activation strategy, the team precisely modulated the hybridization between metal 3d orbitals and oxygen 2p orbitals in the adsorption microenvironment. This approach significantly enhanced the charge transfer efficiency between oxygen evolution reaction (OER) active centers and reaction intermediates. The related research, titled “Defect-Driven Stepwise Activation of Metal–Organic Frameworks toward Industrial-Level Anion Exchange Membrane Water Electrolysis,” was published in the prestigious journal Angewandte Chemie International Edition.
Additionally, by using magnetic moment as a descriptor to guide catalyst design, the team established a correlation between the adsorption energy of chloride ions (Cl⁻) and the binding energies of related reaction intermediates. This led to the successful development of high-efficiency, wide-temperature-range seawater electrolysis. The corresponding work, “Magnetic Moment Descriptor-Guided Multifunctional Co Single-Atom Catalysts Enable Wide- Temperature Uninterrupted Seawater Splitting,” was published in Advanced Materials.
Developing clean and efficient energy conversion technologies such as (seawater) electrolysis for hydrogen production is vital for building a sustainable hydrogen economy. Key electrode reactions involved—OER and the oxygen reduction reaction (ORR)—are complex multi-proton, multi-electron processes with sluggish kinetics, which remain a major bottleneck for overall system efficiency. Therefore, the development of low-cost, efficient, and stable electrocatalysts is urgent. Although noble-metal-based catalysts (e.g., Pt/C and RuO₂) offer excellent intrinsic activity, their scarcity, high cost, and limited stability hinder broader application in seawater electrolysis.
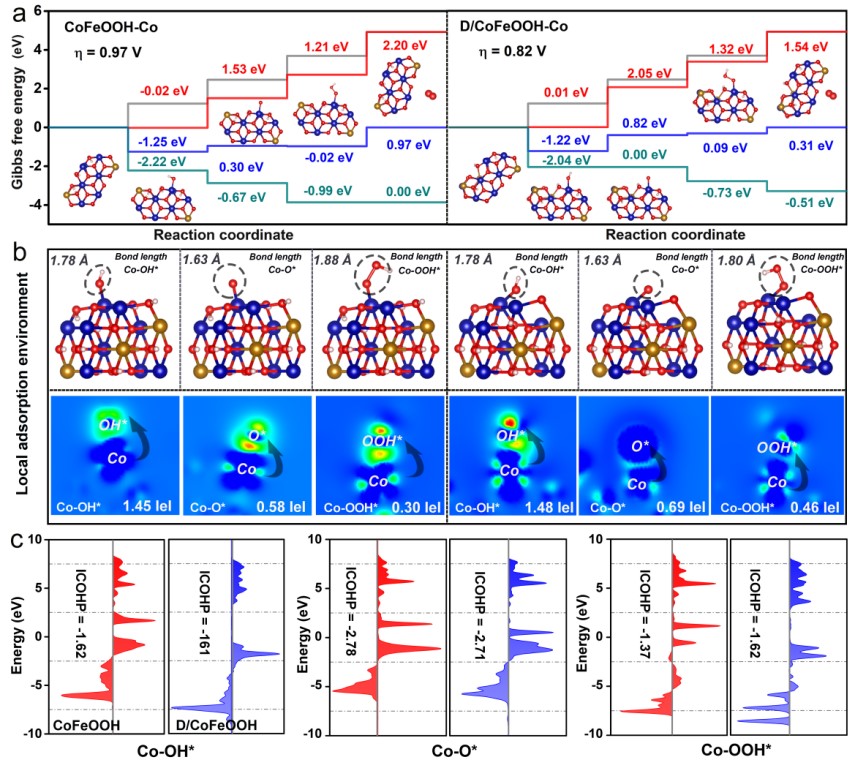
Figure 1. Local Coordination Environment and Electronic Structure of the D-CoFc-MOF Catalyst
To address these challenges, Professor Huang’s team developed a defect-engineered cobalt-based metal-organic framework (D-CoFc-MOF) by modulating the ratio of bridging ligands ferrocene dicarboxylic acid (Fc) and its derivative (Fc′) in coordination with cobalt ions. During OER, the catalyst exhibited a dynamic transformation from the initial MOF structure through an intermediate α-FeOOH phase, ultimately forming a highly active CoFeOOH phase. Theoretical calculations and in situ spectroscopic analyses revealed that introducing defects effectively regulated the local coordination environment and electronic structure, significantly enhancing the adsorption strength of the active Co sites toward the key OOH* intermediate, and reducing the reaction energy barrier of the rate-determining step. An anion exchange membrane water electrolyzer (AEMWE) assembled with this catalyst operated stably for over 300 hours at 60°C and a current density of 1 A/cm², demonstrating excellent catalytic activity and long-term stability.
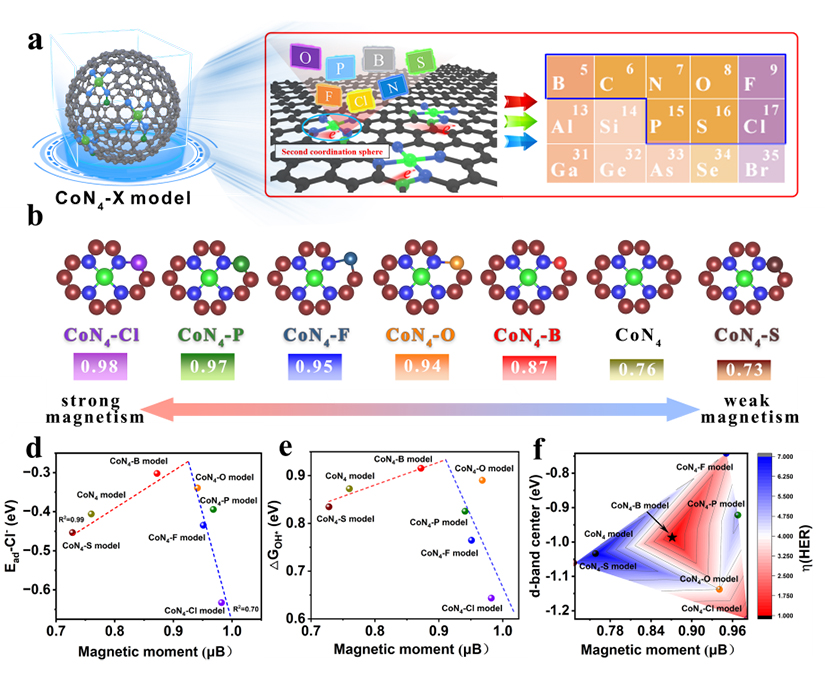
Figure 2. Magnetic Moment Descriptor-Guided Catalyst Design and Adsorption Energy Relationship for Reaction Intermediates
To address the corrosion challenges posed by chloride ions (Cl⁻) in complex seawater electrolysis environments, the team employed magnetic moment descriptors to guide catalyst screening and design. Through second-shell anion engineering, they tuned the coordination environment of Co single-atom sites in CoN₄ structures (CoN₄–X, where X = B/O/F/P/S/Cl). Density functional theory calculations showed that coordination with X anions precisely modulated the spin state of CoN₄ centers, establishing a volcano-type relationship between magnetic moment, Cl⁻ adsorption energy, and the adsorption energies of catalytic intermediates. The CoN₄–B configuration, located at the apex of the volcano curve, significantly reduced Cl⁻ adsorption energy and demonstrated outstanding catalytic performance and corrosion resistance in seawater electrolysis. Remarkably, this catalyst also enabled continuous hydrogen production across a wide temperature range from –30°C to 60°C, achieving a maximum hydrogen production rate of 853μmol/h.
These studies deepen the understanding of structure–activity relationships in metal-organic framework materials and expand the design principles of transition metal single-atom catalysts. The results provide strong support for the continued development of seawater electrolysis technologies for hydrogen production. The research was supported by the National Natural Science Foundation of China, international collaboration projects, and the Natural Science Foundation of Shandong Province. It was conducted in collaboration with the Qingdao Institute of Bioenergy and Bioprocess Technology and the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Ocean University of China is the lead corresponding institution.
Correspondent: Zhang Canhui
Article Links:
https://doi.org/10.1002/adma.202504476
https://doi.org/10.1002/anie.202

